Post-Market Solutions
RegHero’s Post-Market Copilot™ empowers MedTech teams to monitor device safety, filter adverse events from unstructured data, and generate ready-to-submit MDR reports — all with AI-driven precision.

AI-Powered Adverse Event Detection
Automatically extract and identify adverse events from emails, call center logs, chat transcripts, and complaint reports.
Smart Case Triage & Prioritization
Automated routing of cases to quality, clinical, or regulatory teams.
Automated Regulatory Reporting
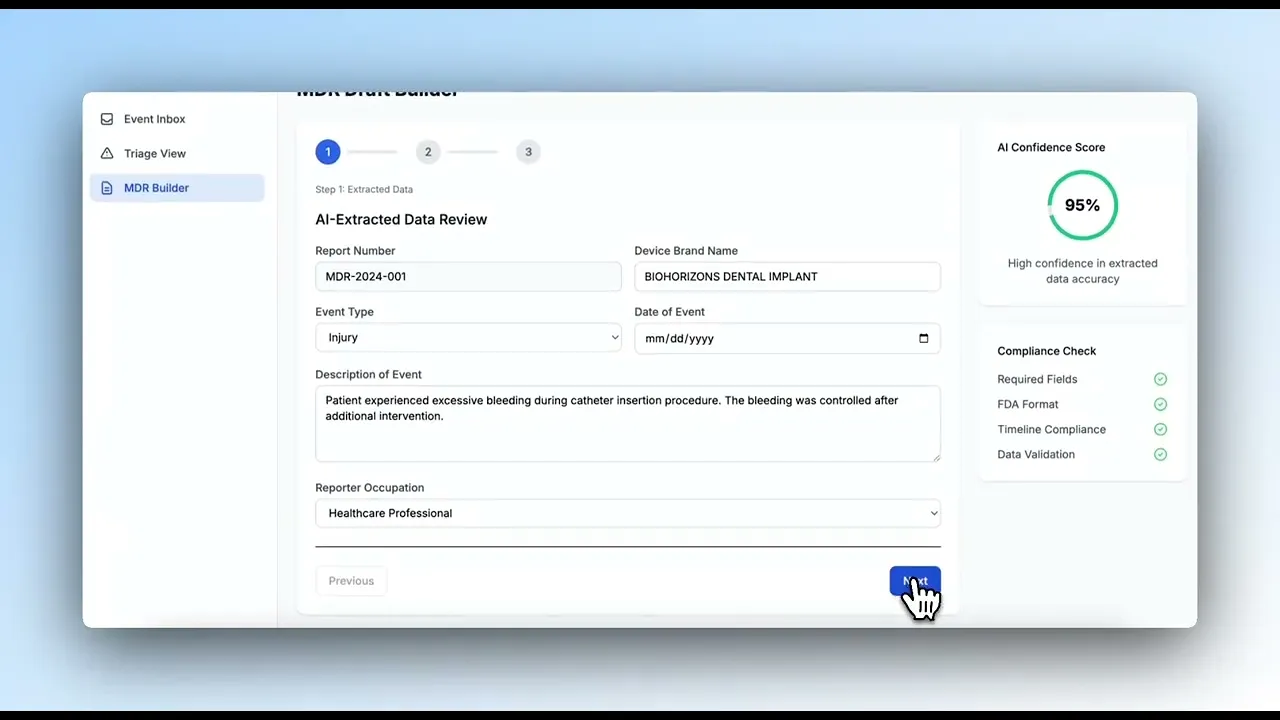
Generate ready-to-submit MDR (Medical Device Reports) for FDA
Post-Market Solutions
RegHero’s Post-Market Copilot™ empowers MedTech teams to monitor device safety, filter adverse events from unstructured data, and generate ready-to-submit MDR reports — all with AI-driven precision.

AI-Powered Adverse Event Detection
Automatically extract and identify adverse events from emails, call center logs, chat transcripts, and complaint reports.
Smart Case Triage & Prioritization
Automated routing of cases to quality, clinical, or regulatory teams.
Automated Regulatory Reporting
Generate ready-to-submit MDR (Medical Device Reports) for FDA
Post-Market Solutions

AI-Powered Adverse Event Detection
Automatically extract and identify adverse events from emails, call center logs, chat transcripts, and complaint reports.
Smart Case Triage & Prioritization
Automated routing of cases to quality, clinical, or regulatory teams.
Automated Regulatory Reporting
Generate ready-to-submit MDR (Medical Device Reports) for FDA

© 2025, RegHero Inc.
© 2025, RegHero Inc.
© 2025, RegHero Inc.